Experiment 3: Esterification
Introduction:
Benzocaine is the trade name for the local anesthetic known chemically as ethyl p-aminobenzoate, which is found in many creams and ointments used in the treatment of pain due to sunburn, minor burns, cuts, scrapes and insect bites. Benzocaine is structurally analogous to Cocaine, Lidocaine, and Novocaine (shown below) and is prepared by the esterification of p-aminobenzoic acid with ethanol.1 Note that esterification reactions can be significantly influenced by the concentration of starting materials and products in solution, as explained by Le Chatelier’s Principle.

1. Salkaowski Ber. 1895, 28, 1921 and Adams, Cohen, Org. Syn. Coll. Vol. I, 2nd ed., 1932, p. 240
Reaction Scheme:
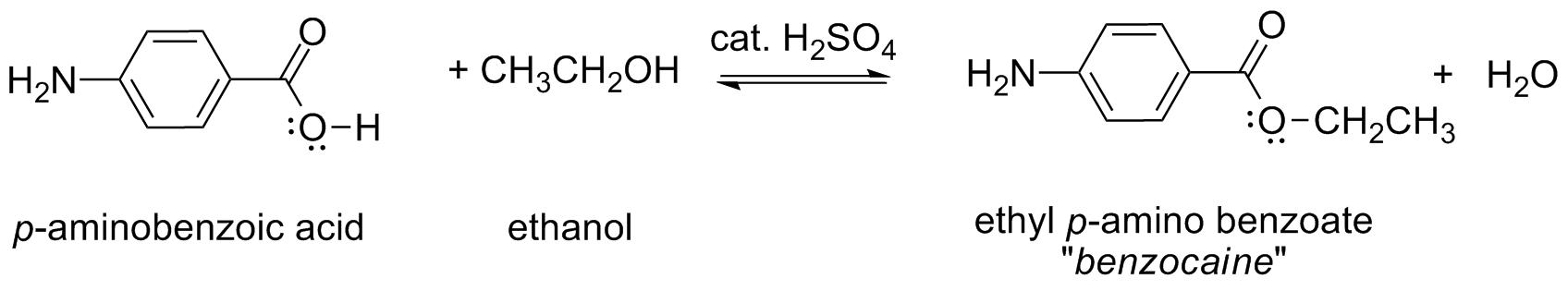
Techniques used: reflux, melting point, recrystallization, gravity & vacuum filtration, thin layer chromatography, and infrared spectroscopy.
Objective: Experiment 3 is a group experiment. You will work in a small group to design and carry out experiments in an attempt to answer one of the focus questions below. Your objective is to form and test a hypothesis in response to the focus question. The general procedure is provided below and should be adapted appropriately.
Focus Questions:
Choose one of the focus questions below.
1. What are the minimum molar equivalents of ethanol that can be used in the esterification reaction and still obtain benzocaine product?(review Le Chatelier’s Principle)
2. What are the maximum molar equivalents of water that can be present in the esterification reaction and still obtain benzocaine product? (review Le Chatelier’s Principle)
3. Can the concentration of para-aminobenzoic acid be increased, rather than ethanol, to increase the quantity of benzocaine product obtained from the reaction?(review Le Chatelier’s Principle)
4. What is the effect if a sterically bulky alcohol, such as isopropanol, is used rather than ethanol in the esterification reaction?
Creativity is encouraged. If you have a different idea for a focus question please discuss it with your GSI before proceeding.
Before lab discuss your focus question with your small group and answer the questions found on the group experimental design sheet at the link here. The sheet is also provided in the resources folder on CTools.
Procedure:
Reagents:
p-aminobenzoic acid
ethanol
concentrated H2SO4
10% sodium carbonate solution
Place 0.360 g of p-aminobenzoic acid and 3.60 mL of ethanol in a 10-mL conical flask. Add a magnetic spin vane and stir the mixture to dissolve the solid acid. With stirring, add 0.30 mL of concentrated sulfuric acid dropwise (CAUTION! sulfuric acid reacts violently with water, is a corrosive, and causes severe burns). Addition of sulfuric acid causes precipitation of the hydrogen sulfate salt of p-aminobenzoic acid, however this precipitate will dissolve during the following reflux period as the hydrogen sulfate salt of the acid is converted into the hydrogen sulfate salt of the ester. To prepare for reflux, attach a water-cooled condenser and heat the mixture so that it boils gently for 60-75 minutes. (Note the quantities here may need to be adjusted depending on your plans be sure to check all calculations with your GSI if you are unsure)
Depending on how you’ve altered the reaction conditions the reaction may take more or less time. You can monitor the progress of the reaction by TLC. To collect a small sample from the refluxing mixture carefully lift up the drying tube at the top of the reflux condenser and lower a long glass pipette down into the mixture. There is no need to use a bulb to withdraw the solution, since a small drop of the reaction mixture will be naturally wick up into the end of the pipette. Use the pipette directly to spot your TLC plate.
When the reaction is complete, remove the reaction apparatus from the heat and allow it to cool. Using a Pasteur pipet, transfer the solution to a small Erlenmeyer flask containing about 3 mL of water. The reaction mixture should dissolve completely in the water, since the ethyl p-aminobenzoate is in the form of the hydrogen salt. After the solution has cooled to room temperature, add the 10% sodium carbonate solution dropwise to neutralize the cooled reaction mixture. As the pH of the solution rises carbon dioxide will be produced and evolve as gas bubbles out of the solution. Continue to add the sodium carbonate until the gas evolution ceases and the pH is above 8. At this point the sulfuric acid is completely neutralized, and the ethyl p-aminobenzoate precipitates.
Collect the precipitated crude ethyl p-aminobenzoate by vacuum filtration using a Hirsch funnel. Use additional portions of water to aid in transferring the crystals to the funnel and wash the crystals with water on the funnel. Dry the crystals overnight and determine the melting point. Pure Benzocaine melts at 92 °C (here you can save a small sample to dry overnight for determination of the melting point and proceed with recrystallization of the bulk of the sample immediately). The crude product can be recrystallized from a mixture of ethanol and water.
Characterization:
To be determined by each lab group as appropriate. Consult your GSI if you are unsure.
Post-lab Questions (write your answer at the end of your lab notebook pages for this experiment):
1. Why is it best to use concentrated H2SO4? Why not use a more dilute solution?
2. What alcohol would you start with if you wanted to prepare Novocaine instead of Benzocaine?
3. What would you do differently if you could modify the design of your experiment?
4. Was your hypothesis correct? (it’s OK if it wasn’t) What results either supported or refuted your hypothesis?
Comments (0)
You don't have permission to comment on this page.